Synthesis of a large library of macrocyclic peptides containing multiple and diverse N-alkylated residues†
Abstract
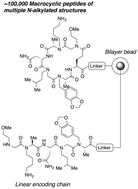
Large combinatorial libraries of macrocyclic peptides are a useful source of bioactive compounds. However, peptides are not generally cell permeable, so there is great interest in the development of methods to create large libraries of modified peptides. In particular, N-alkylation of peptides is known to improve their bioavailability significantly. Incorporation of some level of N-methylated amino acids into peptide libraries has been accomplished with ribosome display or related methods, but the modest efficiency and the inability to employ more diverse N-alkylated amino acids in this type of system argue for the development of synthetic libraries. Here we present optimized procedures for synthesizing macrocyclic peptides containing multiple N-alkylated units and show that this chemistry is efficient enough for the creation of high quality combinatorial libraries by split and pool solid-phase synthesis.
- This article is part of the themed collections: Chemical Biology in Molecular BioSystems and 10th Anniversary of Molecular BioSystems

 Please wait while we load your content...
Please wait while we load your content...