Determination of the binding sites and binding constants between Pb(ii) and DNA using capillary electrophoresis combined with electrothermal atomic absorption spectrometry
Abstract
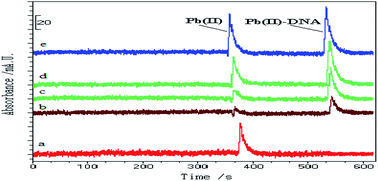
A new method for studying the interaction of lead(II) and DNA was developed using capillary electrophoresis online coupled with electrothermal atomic absorption spectrometry. Under optimized experimental conditions, the detection limit (3σ) for Pb(II) was observed to be 1.8 × 10−6 mol L−1 by running 10 replicates of the reagent blank. The relative standard deviation (RSD, n = 5) of 0.5 × 10−4 mol L−1 was calculated as 3.4%. The primary binding number (n1) and binding constant (K1) of Pb(II)–DNA were observed as 0.57 and 6.2 × 104 L mol−1, respectively. The non-specific binding number (n2) and binding constant (K2) of Pb(II) and DNA were found to be 1.1 and 1.9 × 104 L mol−1, respectively. This new method allows the rapid analysis of a small amount of sample in a simple way, while it also prevents long periods of dialysis and eliminates the interferences from other metal ions. It thus provides a reliable and convenient new way to study the interactions between metal ions and biomolecules.
- This article is part of the themed collection: Analytical atomic spectrometry in China

 Please wait while we load your content...
Please wait while we load your content...