Invariants reveal multiple forms of robustness in bifunctional enzyme systems†
Abstract
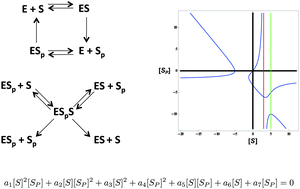
Experimental and theoretical studies have suggested that bifunctional enzymes catalyzing opposing modification and demodification reactions can confer steady-state concentration robustness to their substrates. However, the types of robustness and the biochemical basis for them have remained elusive. Here we report a systematic study of the most general biochemical reaction network for a bifunctional enzyme acting on a substrate with one modification site, along with eleven sub-networks with more specialized biochemical assumptions. We exploit ideas from computational algebraic geometry, introduced in previous work, to find a polynomial expression (an invariant) between the steady state concentrations of the modified and unmodified substrate for each network. We use these invariants to identify five classes of robust behavior: robust upper bounds on concentration, robust two-sided bounds on concentration ratio, hybrid robustness, absolute concentration robustness (ACR), and robust concentration ratio. This analysis demonstrates that robustness can take a variety of forms and that the type of robustness is sensitive to many biochemical details, with small changes in biochemistry leading to very different steady-state behaviors. In particular, we find that the widely-studied ACR requires highly specialized assumptions in addition to bifunctionality. An unexpected result is that the robust bounds derived from invariants are strictly tighter than those derived by ad hoc manipulation of the underlying differential equations, confirming the value of invariants as a tool to gain insight into biochemical reaction networks. Furthermore, invariants yield multiple experimentally testable predictions and illuminate new strategies for inferring enzymatic mechanisms from steady-state measurements.
- This article is part of the themed collection: Integrative Approaches for Signalling and Metabolic Networks

 Please wait while we load your content...
Please wait while we load your content...