One-pot highly diastereoselective annulation to N-unprotected tetrasubstituted 2-pyrrolines†
Abstract
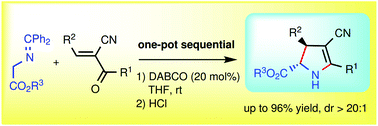
A one-pot DABCO-catalysed Michael addition of glycine imine-derived esters to trans-2-aroyl-3-arylacrylonitriles followed by a deprotection/cyclization/tautomerization sequence afforded tetrasubstituted N-unprotected trans-2-pyrrolines in up to 96% yield.
- This article is part of the themed collection: Elemental Recovery and Sustainability

 Please wait while we load your content...
Please wait while we load your content...