Specificity of the Zn2+, Cd2+ and Ni2+ ion binding sites in the loop domain of the HypA protein†
Abstract
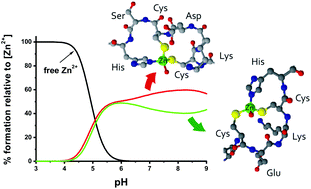
The zinc binding loop domain of the HypA protein of Helicobacter pylori consists of two CXXC motifs with flanking His residues. These motifs bind metal ions, and thus they are crucial for the functioning of the whole protein. The N-terminal site, where His is separated from CXXC by Ser residue is more effective in binding Zn2+ and Ni2+ ions than the C-terminal site, in which His is adjacent to the CXXC motif. Studies on various modifications of the peptide sequence within the Ac-ELECKDCSHVFKPNALDYGVCEKCHS-NH2 loop show the role of the residues in the linker between the CXXC motifs and the effect of the length of the linker on the stability of the complexes it forms with Zn2+, Cd2+ and Ni2+ ions. The proline residue in the linker between the two CXXC binding sites plays a distinct role in the metal ion binding ability of the loop, lowering the efficacy of the metal ion coordination. The deletion of the aliphatic residues from the linker between the CXXC motifs remarkably improves the binding efficacy of the loop.

 Please wait while we load your content...
Please wait while we load your content...