CS2 activation at uranium(iii) siloxide ate complexes: the effect of a Lewis acidic site†
Abstract
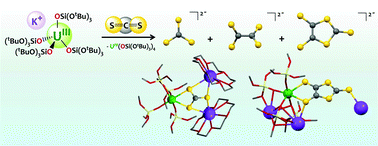
Multimetallic cooperative binding of heteroallenes provides an attractive route to their activation, but the reduction of CS2 at heterobimetallic sites, associating an electron-rich metal with a main group Lewis acid has not been explored. Here we show that the presence of a heterometallic U, K site plays an important role in the CS2 reduction by uranium(III) complexes of the electron-rich and the sterically demanding tris(tert-butoxy)siloxide ligand. Specifically, the ion-pair complex [K(18c6)][U(OSi(OtBu)3)4], 1, leads preferentially to the reductive disproportionation of CS2 to K2CS3 and CS. The crystal structure of the thiocarbonate intermediate complex [U(OSi(OtBu)3)4 (μ3−κ2:κ2:κ2−CS3)K2(18c6)2], 3, isolated from the toluene reaction mixture has been determined. In contrast, the heterobimetallic complex [U(OSi(OtBu)3)4K], 2, promotes preferentially the reductive dimerization of CS2 to K2C2S4 and K2C3S5. The [K2C2S4(DMSO)3]n, 5, and [U(OSi(OtBu)3)4K2(C3S5)]n, 6, polymeric compounds were isolated from this reaction and structurally characterized.
- This article is part of the themed collection: Dalton Discussion 14: Advancing the chemistry of the f-elements

 Please wait while we load your content...
Please wait while we load your content...