Mode-specific fragmentation of amino acid-containing clusters†
Abstract
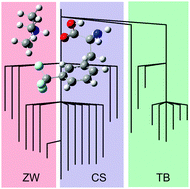
A combination of infrared multiple photon dissociation (IRMPD) spectroscopy and density functional theory calculations have been employed to study the structures and mode-specific dissociation pathways of the proton-bound dimer of 3-trifluoromethylphenylalanine (3-CF3-Phe) and trimethylamine (TMA). Three structural motifs are identified: canonical (charge-solvated), zwitterionic (charge-separated), and TMA-bridged. In the 1000–1350 cm−1 region, similar spectra are observed in the TMA·H+ and 3-CF3-Phe·H+ product channels. At wavenumbers above 1350 cm−1, infrared excitation of charge-solvated structures leads exclusively to production of protonated TMA, while excitation of zwitterionic or TMA-bridged structures results exclusively in production of protonated 3-CF3-Phe. The cluster potential energy landscape is topologically mapped and mechanisms for isomerization and mode-selective dissociation are proposed. In particular, cluster transparency as a result of IR-induced isomerization is implicated in deactivation of some IRMPD channels.

 Please wait while we load your content...
Please wait while we load your content...