N-confused porphyrin tautomers: lessons from density functional theory†
Abstract
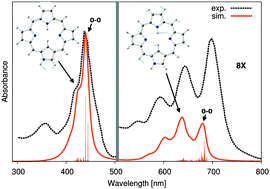
Using first-principle calculations, we characterize the properties of N-confused porphyrins (NCP), with a focus on the differences between the 2H and 3H tautomers. We find that NCP-3H is almost as strongly aromatic as porphyrin, and about twice as aromatic, i.e., remarkably more stable, than NCP-2H, due to the less efficient π-conjugation in the latter form. The deprotonation of the NH-group at the external side of the inverted ring of NCP-2H, adds a lone pair to the π-system, which restores a strong aromaticity, while methylation has no significant effect. Investigating the impact of solvation using a continuum model, we find quite stable solvation energies with a relative dielectric constant, εr, in the 5–40 range, for both tautomers. NCP-3H presents a slightly lower energy than its NCP-2H counterpart in all solvents. However, the energy differences between the two species are of the order of the error margin of the method, hence too small to discuss the experimentally observed stabilization of NCP-3H in dichloromethane (DCM, a poorly polar solvent) and NCP-2H in N,N-dimethylformamide (DMF, a strongly polar solvent) or to extract the population ratios between the two forms in the different solvents. Therefore, the vibronic absorption spectra are also investigated in an effort to rationalize the complex absorption profiles of these NCP derivatives. We find very distinct spectra for the 2H and 3H forms in DMF and DCM, respectively, each fairly reproducing the experiment. We also find that, in the same solvent, the two species exhibit very different signatures, which allows us to conclude that the 2H and 3H tautomers are largely dominant in DMF and DCM, respectively. Interestingly, the vibrational motions that strongly participate in the shoulder of the Soret band and the multiple maxima of the Q-bands largely differ in the two tautomers.

 Please wait while we load your content...
Please wait while we load your content...