Thermodynamics by synchrotron X-ray diffraction: phase relationships and crystal structure of l-tyrosine ethyl ester form III†
Abstract
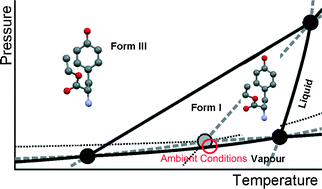
In the case of small organic molecules, phase behaviour, which is important for pharmaceutical applications, is often only studied as a function of temperature. However, for a full thermodynamic description, not only the temperature but also the pressure should be taken into account, because pressure and temperature are the two characteristic variables for the Gibbs energy. The commercial form of L-tyrosine ethyl ester has been studied by synchrotron X-ray diffraction while subjected to different pressures and temperatures. At room temperature, it turns into a new previously unknown form around 0.45 GPa. The structure has been solved with an orthorhombic unit cell, space group P212121, with parameters a = 12.655(4) Å, b = 16.057(4) Å, c = 5.2046(12) Å, and V = 1057.6(5) Å3 at T = 323 K and P = 0.58 GPa. The enthalpy of the transition from the commercial form to the new form could be estimated from the slope of the transition obtained from the synchrotron diffraction data. In addition, the topological pressure–temperature phase diagram has been constructed involving the two solid phases, the liquid and the vapour phase. The solid phases are enantiotropic under low pressure, but the system becomes monotropic at high pressure with the new solid phase being the only stable one.

 Please wait while we load your content...
Please wait while we load your content...