Synthesis and discovery of phytofurans: metabolites of α-linolenic acid peroxidation†
Abstract
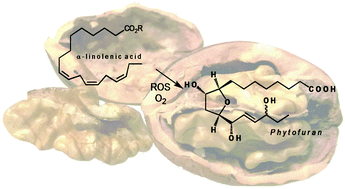
Phytofurans are novel metabolites produced by non-enzymatic peroxidation of α-linolenic acid. An unprecedented Payne rearrangement-cyclization of a C2-symmetric bis-epoxide permitted construction of the core 3-hydroxy-2,5-disubstituted tetrahydrofuran. LC-MS/MS investigation provided evidence for the presence of phytofurans in nuts and seeds for the first time.

 Please wait while we load your content...
Please wait while we load your content...