Total synthesis of (−)-exiguolide via an organosilane-based strategy†
Abstract
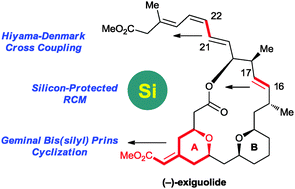
An organosilane-based strategy has been used to accomplish the convergent total synthesis of (−)-exiguolide. The key steps involve: (1) geminal bis(silyl) Prins cyclization to construct the A ring; (2) silicon-protected RCM reaction to construct the 20-membered macrocycle; and (3) Hiyama–Denmark cross-coupling of vinylsilane with vinyliodide to install the triene side chain.

 Please wait while we load your content...
Please wait while we load your content...