From molecular copper complexes to composite electrocatalytic materials for selective reduction of CO2 to formic acid†
Abstract
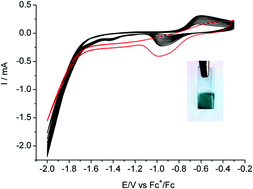
The development of new energy storage technologies is central to solving the challenges facing the widespread use of renewable energies. An option is the reduction of carbon dioxide (CO2) into carbon-based products which can be achieved within an electrochemical cell. Future developments of such processes depend on the availability of cheap and selective catalysts at the electrode. Here we show that a unique well-characterized active electrode material can be simply prepared via electrodeposition from a molecular copper complex precursor. The best performances, namely activity (150 mV onset overpotential and 1 mA cm−2 current density at 540 mV overpotential), selectivity (90% faradaic yield) and stability for electrocatalytic reduction of CO2 into formic acid in DMF/H2O (97 : 3 v/v) have been obtained with the [Cu(cyclam)](ClO4)2 complex (cyclam = 1,4,8,11-tetraazacyclotetradecane) as the precursor. Remarkably the organic ligand of the Cu precursor remains part of the composite material and the electrocatalytic activity is greatly dependent on the nature of that organic component.

 Please wait while we load your content...
Please wait while we load your content...