Superiority of the bi-phasic mixture of a tin-based alloy nanocomposite as the anode for lithium ion batteries†
Abstract
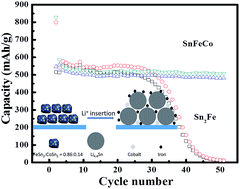
The nanostructured mixture of a Sn2Fe and Sn2Co alloy composite with uniform cubic shaped particles has been synthesized by a reduction-thermal diffusion alloying reaction. The textural properties of the as-prepared samples were characterized by field-emission scanning electron microscopy, transmission electron microscopy and powder X-ray diffraction. Compared with the Sn2Fe alloy, the alloy composite exhibits better reversibility and cycle performance. At a charge/discharge current density of 50 mA g−1, a reversible capacity of 510 mA h g−1 can be maintained after 50 cycles, and its capacity in the 50th cycle was retained at ca. 85% of that in the second cycle. When the current density is increased to 1000 mA g−1, a reversible capacity of 443 mA h g−1 can be still obtained. The ab initio simulation results indicate that Sn2Fe and Sn2Co have similar crystal structures, demonstrating that the two kinds of alloys can be mixed uniformly by the thermal diffusion alloying reaction. The superior electrochemical performance can be attributed to the homogeneously dispersed inactive metallic material (Fe and Co) nanostructure, which partly accommodates the volume change and also retains the integrity of the active material and matrix, resulting in good cycle performance of the composite electrode.

 Please wait while we load your content...
Please wait while we load your content...