Self-healing hydrogels containing reversible oxime crosslinks†
Abstract
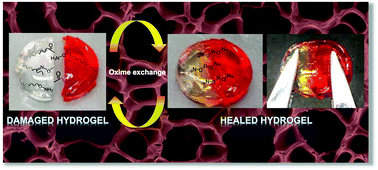
Self-healing oxime-functional hydrogels have been developed that undergo a reversible gel-to-sol transition via oxime exchange under acidic conditions. Keto-functional copolymers were prepared by conventional radical polymerization of N,N-dimethylacrylamide (DMA) and diacetone acrylamide (DAA). The resulting water soluble copolymers (P(DMA-stat-DAA)) were chemically crosslinked with difunctional alkoxyamines to obtain hydrogels via oxime formation. Gel-to-sol transitions were induced by the addition of excess monofunctional alkoxyamines to promote competitive oxime exchange under acidic conditions at 25 °C. The hydrogel could autonomously heal after it was damaged due to the dynamic nature of the oxime crosslinks. In addition to their chemo-responsive behavior, the P(DMA-stat-DAA) copolymers exhibit cloud points which vary with the DAA content in the copolymers. This thermo-responsive behavior of the P(DMA-stat-DAA) was utilized to form physical hydrogels above their cloud point. Therefore, these materials can either form dynamic-covalent or physically-crosslinked gels, both of which demonstrate reversible gelation behavior.

 Please wait while we load your content...
Please wait while we load your content...