Aggregation behavior of a gemini surfactant with a tripeptide spacer†
Abstract
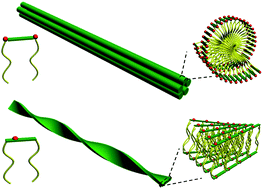
A peptide gemini surfactant, 12-G(NH2)LG(NH2)-12, has been constructed with two dodecyl chains separately attached to the two terminals of a glutamic acid–lysine–glutamic acid peptide and the aggregation behavior of the surfactant was studied in aqueous solution. The 12-G(NH2)LG(NH2)-12 molecules form fiber-like precipitates around pH 7.0, and the precipitation range is widened on increasing the concentration. At pHs 3.0 and 11.0, 12-G(NH2)LG(NH2)-12 forms soluble aggregates because each molecule carries two positively charged amino groups at the two ends of the peptide spacer at pH 3.0, while each molecule carries one negatively charged carboxyl group in the middle of the peptide spacer at pH 11.0. 12-G(NH2)LG(NH2)-12 displays a similar concentration-dependent process at these two pHs: forming small micelles above the critical micelle concentration and transferring to fibers at pH 3.0 or twisted ribbons at pH 11.0 above the second critical concentration. The fibers formed at pH 3.0 tend to aggregate into bundles with twisted structure. Both the twisted fibers at pH 3.0 and the twisted ribbons at pH 11.0 contain β-sheet structure formed by the peptide spacer.

 Please wait while we load your content...
Please wait while we load your content...