Atomic-scale cation dynamics in a monolayer VOX/α-Fe2O3 catalyst†
Abstract
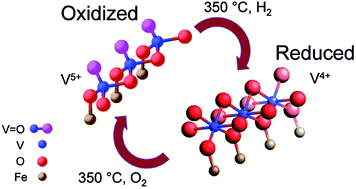
Catalytic reactions are associated with dynamical changes in the catalyst that include the oxidation state and local structural variations. The understanding of such dynamics, particularly at the atomic-scale, is of great importance for revealing the activity and selectivity of the catalyst in numerous reactions. Combining in situ X-ray absorption spectroscopy with in situ diffuse reflectance infrared Fourier transform spectroscopy, we studied the redox-induced dynamical changes for a VOX monolayer catalyst supported on a α-Fe2O3 powder. The results show that several co-existing VOX species reversibly change their V oxidation states between +5 and +4, in concurrence with a structural change from two-dimensional chains to well connected V–O–V networks. These changes are also associated with the breaking and formation of the V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vanadyl group. This combined study provides new insight into how VOX species change during catalytic reactions, which leads to proposed atomic-scale models for the redox-induced dynamics of the catalyst.
O vanadyl group. This combined study provides new insight into how VOX species change during catalytic reactions, which leads to proposed atomic-scale models for the redox-induced dynamics of the catalyst.


 Please wait while we load your content...
Please wait while we load your content...