Facile synthesis of Mn6.87(OH)3(VO4)3.6(V2O7)0.2 microtubes and their application as an anode material for lithium-ion batteries†
Abstract
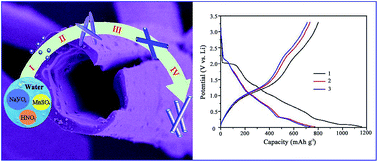
Mn6.87(OH)3(VO4)3.6(V2O7)0.2 microtubes were successfully synthesized by a simple and facile hydrothermal method without using any surfactants or additives. The reaction conditions influencing the structures and morphologies of the products, such as reaction time, reaction temperature, and pH value, were systematically investigated. An Ostwald ripening–dissolution–recrystallization process was proposed to elucidate the formation of the tube-like microstructure. Electrochemical measurements revealed that the as-prepared Mn6.87(OH)3(VO4)3.6(V2O7)0.2 microtubes exhibited high specific capacity, high coulombic efficiency, and good cycling stability, indicating a promising anode candidate for application in lithium-ion batteries. Significantly, this is the first report on Mn6.87(OH)3(VO4)3.6(V2O7)0.2 as an anode material for lithium-ion batteries, and the present work will greatly expand the range of anode choices in lithium-ion batteries.

 Please wait while we load your content...
Please wait while we load your content...