Solution processable low bandgap thienoisoindigo-based small molecules for organic electronic devices†
Abstract
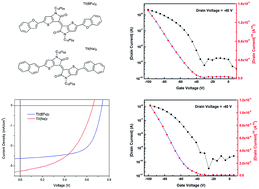
Two donor–acceptor conjugated small molecules that consist of thienoisoindigo as an acceptor unit and benzofuran or naphthalene as a donor building block have been synthesized via a Suzuki coupling reaction. The small molecules, TII(BFu)2 and TII(Na)2, exhibit broad and near-infrared absorption in the range of 500–900 nm while their absorption maxima locate at around 620 nm in films. Optical band gaps calculated from the solid state absorption cutoff value are 1.49 eV for TII(BFu)2 and 1.53 eV for TII(Na)2, respectively. These small molecules possess p-channel charge transport characteristics when used as the active semiconductor in organic thin-film transistors (OFETs). The highest hole mobilities of 1.28 × 10−3 cm2 V−1 s−1 for TII(BFu)2 and 1.29 × 10−3 cm2 V−1 s−1 for TII(Na)2 have been achieved in top-contact-bottom-gate OFET devices. The preliminary characterization of bulk heterojunction photovoltaic devices consisting of small molecules and PC71BM yield power conversion efficiencies (PCE) of 1.24% for TII(BFu)2 and 1.04% for TII(Na)2. Moreover, the relationships between the semiconductor devices performance and film morphology, and energy levels are discussed.

 Please wait while we load your content...
Please wait while we load your content...