The first registration of a green liquid-phase chemiluminescence of the divalent Eu2+* ion in interaction of β-diketonate complexes Eu(acac)3·H2O, Eu(dpm)3, Eu(fod)3 and Eu(CH3COO)3·6H2O with Bui2AlH in THF with the participation of oxygen†
Abstract
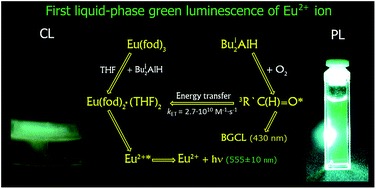
The first liquid-phase green chemiluminescence (CL) of the divalent europium Eu2+* ion in a study of interactions in the systems EuL3·(H2O)x–THF–Bui2AlH–O2 (L = acac, dpm, fod, and CH3COO; x = 0, 1, 6; Bui = iso-C4H9) was discovered. The attack of Bui2AlH on the water of crystallization of complexes EuL3·(H2O)x affords a luminoxane (Bui2Al)2O, hydrogen, and isobutane. The reaction of Bui2AlH and EuL3 moiety of the initial Eu3+ complexes leads to the not previously synthesized divalent europium complexes EuL2·(THF)2, which were isolated and characterized by elemental and spectral analyses in the present work. In the reaction solutions, these complexes are associated into bulky complexes with an excess of diisobutylaluminum hydride, EuL2·(THF)2–Bui2AlH. The measured CL and photoluminescence (PL) spectra of yellow-green reaction solutions in both cases consist of one broad band with λmax = 555 ± 10 nm due to the emission of the electron-excited state of the Eu2+* ion (4f65d1 → 4f7 transition), a part of the bulky complex EuL2·(THF)2–Bui2AlH. The lifetime of the excited states, and PL and CL quantum yields of the Eu2+* ion in the EuL2·(THF)2–Bui2AlH complexes were determined. A mechanism (“indirect CL”) is proposed to explain the generation of the green CL. The primary emitter, triplet-excited isobutyric aldehyde 3R′C(H)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O* (R′ = iso-C3H7), is formed in the disproportionation reaction of peroxyl radicals – intermediates of Bui2AlH oxidation by oxygen. The exited 3R′C(H)
O* (R′ = iso-C3H7), is formed in the disproportionation reaction of peroxyl radicals – intermediates of Bui2AlH oxidation by oxygen. The exited 3R′C(H)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O* molecules transfer energy to Eu2+ ion converting it into the electronically exited state Eu2+*, which is then deactivated by emitting quanta of green light due to the 4f65d1 → 4f7 transition. It was found that for divalent Eu2+ ions, unlike trivalent Ln3+ ions, replacement of the inorganic anion-ligand Cl− (complex EuCl2·(THF)2–Bui2AlH) with organic one L (complex EuL2·(THF)2–Bui2AlH) decreases the CL and PL intensity and leads to the green-shift of the luminescence maximum of Eu2+* from the blue (λmax = 465 nm) to the green (λmax = 555 nm) region. The high brightness and duration of the CL, visible to the naked eye in the system Eu(fod)3–THF–Bui2AlH–O2, make it promising as a chemical source of green light.
O* molecules transfer energy to Eu2+ ion converting it into the electronically exited state Eu2+*, which is then deactivated by emitting quanta of green light due to the 4f65d1 → 4f7 transition. It was found that for divalent Eu2+ ions, unlike trivalent Ln3+ ions, replacement of the inorganic anion-ligand Cl− (complex EuCl2·(THF)2–Bui2AlH) with organic one L (complex EuL2·(THF)2–Bui2AlH) decreases the CL and PL intensity and leads to the green-shift of the luminescence maximum of Eu2+* from the blue (λmax = 465 nm) to the green (λmax = 555 nm) region. The high brightness and duration of the CL, visible to the naked eye in the system Eu(fod)3–THF–Bui2AlH–O2, make it promising as a chemical source of green light.

 Please wait while we load your content...
Please wait while we load your content...