Anion–π interactions in protein–porphyrin complexes
Abstract
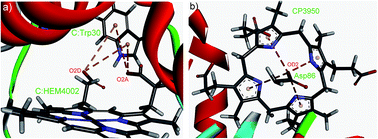
In this work, we have analyzed the influence of anion–π interactions on the stability of high resolution protein–porphyrin complex crystal structures. The anion–π interactions are distance and orientation dependent. Results of ab initio calculations of stabilization energies showed that they lie mostly in the range from −2 to −4 kcal mol−1 with some of the anion–π interactions having stabilization energies of up to −16 kcal mol−1. In the anionic group, the numbers of anion–π interactions involving Asp and Glu are similar, while His is more often involved in these interactions than other aromatic residues. Furthermore, our study showed that in the dataset used about 70% of the investigated anion–π interactions are in fact multiple anion–π interactions. Our results suggest that interacting residues are predominantly located in buried and partially buried regions. The secondary structure of the anion–π interaction involving residues shows that most of the interacting residues preferred to be in helix conformations. Significant numbers of aromatic residues involved in anion–π interactions have one or more stabilization centers, providing additional stability to the protein–porphyrin complexes. The conservation patterns indicate that more than half of the residues involved in these interactions are evolutionarily conserved, indicating that the contribution of the anion–π interaction is an important factor for the structural stability of the investigated protein–porphyrin complexes.

 Please wait while we load your content...
Please wait while we load your content...