Highly selective zirconia-based propene sensor attached with sol–gel derived NiO nanospheres
Abstract
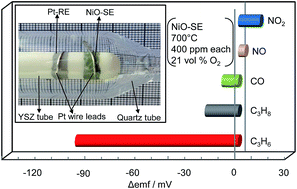
Nanospheres of nickel oxide (NiO) were synthesized by a simple modified sol–gel route using nickel nitrate as a precursor and citric acid as a gelling agent. An yttria-stabilized zirconia (YSZ)-based sensor was fabricated using the sol–gel derived NiO nanospheres as the sensing electrode (SE) and Pt as the reference electrode (RE), and its sensing characteristics were examined in the temperature range of 650–800 °C under various O2 concentrations (5, 10, 15 and 21 vol%). The fabricated NiO-SE was characterized by X-ray diffraction (XRD) and scanning electron microscopy (SEM). It turned out that the sensor attached with the sol–gel derived NiO-SE exhibited a selective and sensitive response to propene (C3H6) at 700 °C in all measured O2 concentrations. The sensor showed the highest sensitivity and selectivity to propene in 21 vol% O2. In addition, the sensor exhibited a stable response to C3H6 for about two months. The sensing mechanism was confirmed to be mixed-potential-type. It is believed that the nanofeatures associated with the sol–gel derived NiO seemed to be responsible for the high sensitivity and selectivity to C3H6.

 Please wait while we load your content...
Please wait while we load your content...