Synthesis of a 2,4,6,8,10-dodecapentanoic acid thioester as a substrate for biosynthesis of heat stable antifungal factor (HSAF)†
Abstract
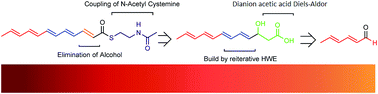
The N-acetylcystamine (SNAC) thioester of dodecapentaenoic acid, an analog of a putative intermediate in the biosynthesis of Heat Stable Antifungal Factor (HSAF), is synthesized. Key steps include sequential Horner–Emmons homologations with the Weinreb amide of diethylphosponoacetic acid, and thioesterification of an aldol-derived 3-hydroxyalkanoate, which serves as a stable precursor of the sensitive polyenoate. The thioester was investigated as a biosynthetic substrate using a purified nonribosomal peptide synthetase and was not incorporated in the observed products.

 Please wait while we load your content...
Please wait while we load your content...