Reductant-triggered rapid self-gelation and biological functionalization of hydrogels†
Abstract
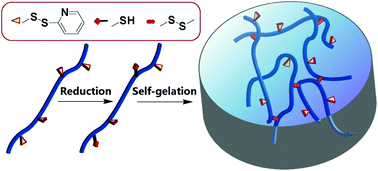
Hydrogels have found wide applications in many fields. Although plenty of mature preparation methodologies are available now, there is certainly a continuous impetus in developing new methods toward simplicity, adjustability and satisfying diverse emerging needs. In this paper, a novel and efficient approach for hydrogel preparation is proposed. Polyacrylamide (PAM) chains with pyridyl disulfide (PDS) side groups were synthesized by free-radical polymerization. Upon addition of a tiny amount of reductants to the aqueous solution of the synthesized copolymer, part of the PDS groups are reduced to free thiols, and the subsequent exchange reaction between thiol groups and PDS results in disulfide-crosslinked hydrogels. The rapid self-gelation process was monitored by in situ rheological measurements, and the network structures of the hydrogels were observed using a scanning electron microscope. It has been found that the mechanical properties and pore sizes of the hydrogels could be tuned by changing the polymer concentration and the amounts of the reductants added into the solution. The residual PDS groups in the hydrogels provide reaction sites for immobilization of biological functional molecules. Reduced glutathione and bovine serum albumin were successfully immobilized in the hydrogels, and the bioactivities of the hydrogels were measured in this research. The disulfide crosslinks are cleavable and the hydrogels are dissociated under reducing conditions, which indicates that the hydrogels may find potential applications in biological and medical fields.

 Please wait while we load your content...
Please wait while we load your content...