Synthesis, characterization, micellization and application of novel multiblock copolymers with the same compositions but different linkages
Abstract
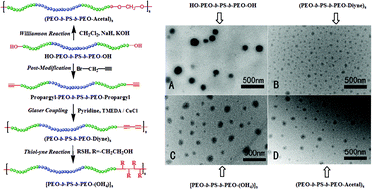
Several novel multiblock copolymers, (PEO-b-PS-b-PEO-Diyne)s, [PEO-b-PS-b-PEO-(OH)4]s and (PEO-b-PS-b-PEO-Acetal)s, with the same compositions but different linkages were constructed, and their micellization and application were studied. First, the precursor HO-PEO-b-PS-b-PEO-OH was prepared by sequential LAP and ROP mechanisms, and the precursor Propargyl-PEO-b-PS-b-PEO-Propargyl was achieved by a subsequent modification procedure. Subsequently, using the efficient Glaser coupling reaction, the multiblock copolymer (PEO-b-PS-b-PEO-Diyne)s was synthesized and the dyine groups embedded in the main chain were modified by a thiol–yne reaction to give multiblock copolymer [PEO-b-PS-b-PEO-(OH)4]s. Also, using the efficient Williamson reaction, the multiblock copolymer (PEO-b-PS-b-PEO-Acetal)s was obtained. Finally, the micellar morphologies formed from the synthesized copolymers were investigated and compared by DLS and TEM measurements, and the in vivo distribution of the micelles was also studied by loading them with a fluorescent probe. The results revealed that, under the same conditions, the multiblock copolymers can form micelles of different sizes. Due to the hydrophobicity of the introduced dyine groups and PS segments, smaller sized micelles can be formed, which traverse the BBB and therefore might result in a therapeutic application in the treatment of brain disease. However, the hydrophilicity of the acetal and hydroxyl groups gave a similar effect to that of the PEO segment, and larger sized micelles were formed.

 Please wait while we load your content...
Please wait while we load your content...