Poly(thiolactone) homo- and copolymers from maleimide thiolactone: synthesis and functionalization†
Abstract
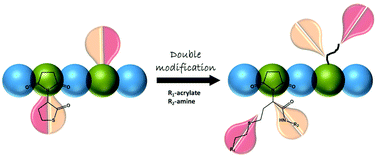
We describe the synthesis of a thiolactone-functionalized maleimide (MITla), and its (co)polymerization into poly(thiolactone) homo- and copolymers via controlled or free radical polymerization (CRP or FRP) techniques. Homopolymers were synthesized using FRP whereas MITla was copolymerized with styrene and N-iso-propylacrylamide (NIPAAm) via RAFT. In that way, we were able to combine the properties of a maleimide with the possibility to use the thiolactone side chain functionality in subsequent double modification reactions. Thiolactones are susceptible to nucleophilic ring-opening in the presence of primary amines, releasing a thiol moiety that can be used for conjugate addition (nucleophilic thiol–ene) reactions afterwards. We synthesized and characterized copolymers of different compositions, followed by site-specific double modification reactions with a combination of n-butylamine and methyl acrylate.

 Please wait while we load your content...
Please wait while we load your content...