Frequency control of crossover reactions in concurrent cationic vinyl-addition and ring-opening copolymerization of vinyl ethers and oxiranes: specific roles of weak Lewis bases and solvent polarity†
Abstract
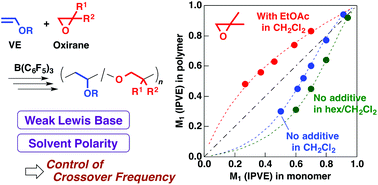
Weak Lewis bases and solvent polarity are demonstrated to be highly responsible for the frequency of crossover reactions in the concurrent cationic vinyl-addition and ring-opening copolymerization of alkyl vinyl ethers (VEs) and oxiranes. Weak Lewis bases such as ethyl acetate and 1,4-dioxane promote crossover reactions from isobutylene oxide- or butadiene monoxide-derived propagating species to an alkyl VE monomer, potentially through the more frequent generation of carbocations via the ring opening of the oxonium ion. The specificity of the carbocation that results from the ring-opening reaction—a preference for VE monomers or an aversion to oxirane monomers—is another factor that changes the crossover frequency. Weak Lewis bases, however, have little effect on the relative reactivity of the VE-derived propagating species to each monomer. In contrast, solvent polarity has a significant effect on the promotion of crossover from the VE-derived propagating end to an oxirane, while the frequency of the crossover from the oxirane-derived propagating end is not affected. In contrast to the two oxiranes, the reaction conditions have little effect on the copolymerization using isoprene monoxide, which is an oxirane that generates a more stable, resonance-stabilized carbocation through ring opening. The relative reactivities of VEs and oxiranes under various conditions are discussed in terms of the average number of each monomer unit in one block of the copolymers and in terms of the monomer reactivity ratios.
- This article is part of the themed collection: Emerging Investigators

 Please wait while we load your content...
Please wait while we load your content...