Participation of an additional 4′-hydroxymethyl group in the cleavage and isomerization of ribonucleoside 3′-phosphodiesters†
Abstract
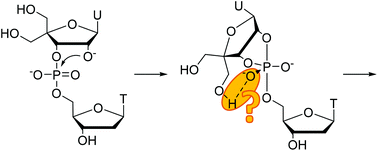
4′-(Hydroxymethyl)uridylyl-3′,5′-thymidine, an RNA model bearing an extra hydroxymethyl group at the 4′-position of the 3′-linked nucleoside, has been prepared and its cleavage and isomerization reactions studied over a wide pH range (from 0 to 12). Overall, the pH-rate profiles of these reactions were very similar to those of uridylyl-3′,5′-uridine (UpU) – only a very modest acceleration was observed under acidic and neutral conditions. Evidently, hydrogen bond assistance by the additional hydroxymethyl function does not play a significant role.


 Please wait while we load your content...
Please wait while we load your content...