Muscarine-like compounds derived from a pyrolysis product of cellulose†
Abstract
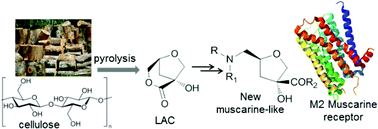
Cellulose represents a key component of a renewable biomass source, from which chiral compounds with a high added value in the application for the synthesis of potentially bioactive molecules can be obtained. The anhydrosugar (1R,5S)-1-hydroxy-3,6-dioxa-bicyclo[3.2.1]octan-2-one (LAC), produced on the gram-scale by catalytic pyrolysis of cellulose, was used as a building block in the synthesis of five new enantiomerically pure muscarine-like products. The structures of the target compounds 4–8 showed different substituents at the C-2 and C-4 positions, but each of them had the same (2S,4R) configuration as the natural (+)-muscarine. A renewed interest in new muscarinic analogues is due to the design and synthesis of molecules exhibiting a higher selectivity for a specific muscarinic receptor and due to the development of effective agents in the treatment of Alzheimer's disease and other cognitive disorders. In this context, products 4–8 were investigated with respect to their binding affinity to human M1–M5 muscarinic acetylcholine receptors. The data indicated that compound 8, emerging as the most active in the series with values comparable to natural (+)-muscarine and a moderate selectivity in favor of the hM2 subtype receptor, also exhibited the highest stability during the interaction with the hM2 (3UON) subtype muscarinic receptor by using a docking calculation.

 Please wait while we load your content...
Please wait while we load your content...