The development and use of a general route to brassinolide, its biosynthetic precursors, metabolites and analogues†
Abstract
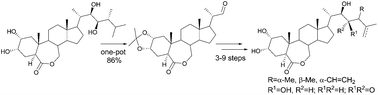
A new method for the construction of steroid side chains through the addition of lithium salts of dithianes to a C-22 aldehyde was developed. An efficient one-pot procedure for the preparation of a suitable C-22 aldehyde from commercial epibrassinolide in three steps in 86% isolated yield was described. Enantioselective hydroxymethylation of isovaleraldehyde and Kulinkovich cyclopropanation of silylated Roche esters were used as key steps for the dithiane syntheses. The method was applied for the preparation of brassinolide, its biosynthetic precursors and metabolites. In addition, a number of brassinosteroids with a double bond in the side chain were prepared as precursors for tritiated derivatives for biosynthetic studies.

 Please wait while we load your content...
Please wait while we load your content...