The fate of nano-silver in aqueous media†
Abstract
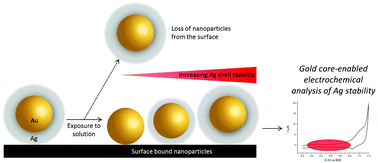
Silver nanoparticles offer highly attractive properties for many applications, however concern has been raised over the possible toxicity of this material in environmental systems. While it is thought that the release of Ag+ can play a crucial role in this toxicity, the mechanism by which the oxidative dissolution of nano-silver occurs is not yet understood. Here we address this through the electrochemical analysis of gold-core silver-shell nanoparticles in various solutions. This novel method allows the direct quantification of silver dissolution by normalisation to the gold core signal. This is shown to be highly effective at discriminating between silver dissolution and the loss of nanoparticles from the electrode surface. We evidence through this rigorous approach that the reduction of O2 drives the dissolution of nano-silver, while in the presence of Cl− this dissolution is greatly inhibited. This work is extended to the single nanoparticle level using nano-impact experiments.

 Please wait while we load your content...
Please wait while we load your content...