Thermodynamic stability of high phosphorus concentration in silicon nanostructures
Abstract
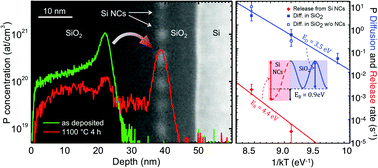
Doping of Si nanocrystals (NCs) has been the subject of a strong experimental and theoretical debate for more than a decade. A major difficulty in the understanding of dopant incorporation at the nanoscale is related to the fact that theoretical calculations usually refer to thermodynamic equilibrium conditions, whereas, from the experimental point of view, impurity incorporation is commonly performed during NC formation. This latter circumstance makes impossible to experimentally decouple equilibrium properties from kinetic effects. In this report, we approach the problem by introducing the dopants into the Si NCs, from a spatially separated dopant source. We induce a P diffusion flux to interact with the already-formed and stable Si NCs embedded in SiO2, maintaining the system very close to the thermodynamic equilibrium. Combining advanced material synthesis, multi-technique experimental quantification and simulations of diffusion profiles with a rate-equation model, we demonstrate that a high P concentration (above the P solid solubility in bulk Si) within Si NCs embedded in a SiO2 matrix corresponds to an equilibrium property of the system. Trapping within the Si NCs embedded in a SiO2 matrix is essentially diffusion limited with no additional energy barrier, whereas de-trapping is prevented by a binding energy of 0.9 eV, in excellent agreement with recent theoretical findings that highlighted the impact of different surface terminations (H- or O-terminated NCs) on the stability of the incorporated P atoms.

 Please wait while we load your content...
Please wait while we load your content...