Improved electrochemical performance of nitrogen doped TiO2-B nanowires as anode materials for Li-ion batteries†
Abstract
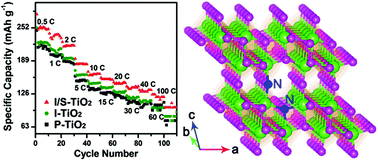
N-doped TiO2-B nanowires are prepared by the solvothermal method using TiN nanoparticles as the starting material. X-ray photoelectron spectroscopy shows that the N dopants preferentially occupy the interstitial sites of TiO2-B up to a content of ∼0.55 at%. Above this critical value, the N dopants will substitute the oxygen atoms which improve the electronic conductivity of TiO2-B. The maximum proportion of substituted-N in the TiO2-B nanowires is ∼1.3 at%. Raman scattering shows that the substituted-N strengthens the Ti(1)–O1–Ti(2) and O1–Ti(1)–O3 bonds of TiO2-B. This improves the stability of the corresponding local structures, thus reducing the distortion of the Li+ diffusion channel along the b-axis of TiO2-B. As a result, the substituted-N has more of an impact on the electrochemical properties of TiO2-B than the interstitial-N does. The TiO2-B nanowires containing substituted-N dopants exhibit a remarkably enhanced electrochemical performance compared to pure TiO2-B. They show a discharge capacity of 153 mA h g−1 at the 20 C rate with a capacity retention of 76% after 1000 cycles. In addition, they can deliver a discharge capacity of 100 mA h g−1 at an ultra-high rate of 100 C, indicating their great potential in high power lithium ion batteries.

 Please wait while we load your content...
Please wait while we load your content...