Mitochondria-acting hexokinase II peptides carried by short-length carbon nanotubes with increased cellular uptake, endosomal evasion, and enhanced bioactivity against cancer cells†
Abstract
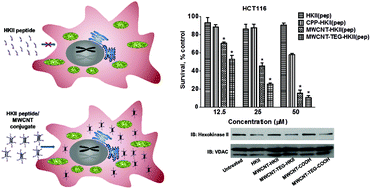
Type II hexokinase (HKII) has emerged as a viable therapeutic target due to its involvement in metabolic reprogramming and also apoptosis prevention. The peptide derived from the fifteen amino acid sequence in the HKII N-terminal region [HKII(pep)] can compete with endogenous proteins for binding on mitochondria and trigger apoptosis. However, this peptide is not cell-permeable. In this study, multi-walled carbon nanotubes (MWCNTs) were used to effectively deliver HKII(pep) across cellular barriers without compromising their bioactivity. The peptide was conjugated on either oxidized MWCNTs or 2,2′-(ethylenedioxy)bis(ethylamine)-functionalized MWCNTs, yielding MWCNT-HKII(pep) and MWCNT-TEG-HKII(pep), respectively. Both conjugates were shown to be internalized by breast cancer MCF-7 cells using confocal microscopy. Moreover, these nanoconjugates seemed to have escaped from endosomes and be in the vicinity of mitochondria. The WST-1 cytotoxicity assay conducted on MCF-7 and colon carcinoma HCT116 cells revealed that MWCNT–peptide conjugates were significantly more effective in curbing cancer cell growth compared to a commercially available cell permeable HKII fusion peptide. In addition, both nanoconjugates displayed an enhanced ability in eliciting apoptosis and depleting the ATP level in HCT116 cells compared to the mere HKII peptide. Importantly, hexokinase II release from mitochondria was demonstrated in MWCNT-HKII(pep) and MWCNT-TEG-HKII(pep) treated cells, highlighting that the structure and bioactivity of HKII(pep) were not compromised after covalent conjugation to MWCNTs.


 Please wait while we load your content...
Please wait while we load your content...