Complexation of some alkali and alkaline earth metal cations by macrocyclic compounds containing four pyridine subunits – a DFT study†
Abstract
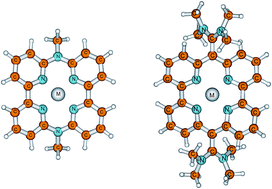
The thermodynamic properties of the complexes of some alkali and doubly-charged alkaline earth metal cations with tetradentate pyridine-based macrocyclic compounds in the gas phase and in acetonitrile solution have been established by quantum chemical calculations. It is shown by the reliable DFT B3LYP/6-311+G(3df,2p)//B3LYP/6-31G(d) method that these supramolecular structures act as efficient and very selective scavengers of Li+, Na+, K+, Be2+, Mg2+ and Ca2+ metal cations, exhibiting gas phase cation affinities in the range between 58.5 and 553.8 kcal mol−1 in the sequence K+ < Na+ < Li+ < Ca2+ < Mg2+ < Be2+. The structures of the uncomplexed and complexed ligands are provided and the changes in the macrocyclic ring conformation and the geometrical response of the ligand binding site to the varying guests have been analyzed. The charge transfer process and the nature of the interactions in the formed complexes have been investigated. The results revealed that metal cations act as charge acceptors and the amount of charge transfer is in agreement with cation affinities. The bond order analysis shows medium strength covalent interactions between the four pyridine nitrogen atoms and the metal cation in complexes with Be2+, while the smallest covalent interaction was found for K+ complexes. The cation affinity of the ligands is amplified by attaching electron-donating substituents to the pyridine rings. The solvent effects were assessed using the polarized continuum method (PCM). The complexation order was preserved in acetonitrile solution.

 Please wait while we load your content...
Please wait while we load your content...