An electrochemical sensor based on rhodamine B hydrazide-immobilized graphene oxide for highly sensitive and selective detection of Cu(ii)†
Abstract
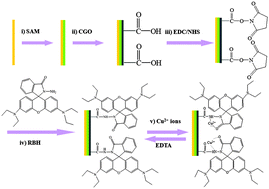
A novel strategy for fabricating a Cu2+ sensor based on rhodamine B hydrazide (RBH)-immobilized graphene oxide (GO) was reported. The thiol-modified Au electrode was functionalized by carboxyl functionalized GO through intermolecular interactions, followed by chemical bonding with RBH. The developed nanocomposite was used as an electrochemical sensor for detecting Cu2+ in aqueous solution using electrochemical impedance spectroscopy analysis with a detection limit of 0.061 nM within the range from 0.1 to 50 nM. Furthermore, the interference from potentially interfering ions such as Hg2+, Ag+, Cr2+, Fe2+, Pb2+, Ba2+, Mn2+, Co2+, and Ni2+ associated with Cu2+ analysis could be effectively inhibited. In addition, the developed Cu2+ sensor could be reproduced up to 10 cycles. In this approach, the fluorescent probe RBH can be replaced by other fluorescein derivatives which could identify the corresponding ions, which makes the approach a widely applicable strategy for metal ion detection.

 Please wait while we load your content...
Please wait while we load your content...