A diaminomaleonitrile based selective colorimetric chemosensor for copper(ii) and fluoride ions†
Abstract
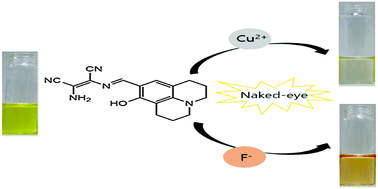
A new and simple colorimetric receptor 1, based on 2,3-diaminomaleonitrile and julolidine moieties, has been synthesized and characterized. 1 showed a selective colorimetric sensing ability for copper(II) ions by changing color from yellow to colorless, and could be utilized to monitor Cu2+ over a wide pH range of 4–12. The detection limit of 1 (2.1 μM) for Cu2+ ions is much lower than that recommended by WHO in drinking water (30 μM). Moreover, the receptor 1 can also detect fluoride by color change from yellow to orange, distinguishing the fluoride ions effectively from anions such as CH3COO− and CN−. It was also found that the 1–F− complex was reversibly bound and could be simply reverted back through treatment with a proper reagent such as HCl. The sensing mechanism for F− was theoretically supported by DFT and TD-DFT calculations.

 Please wait while we load your content...
Please wait while we load your content...