Inorganic layered structures in hybrid double sulfates: related phases and thermal reactivity†
Abstract
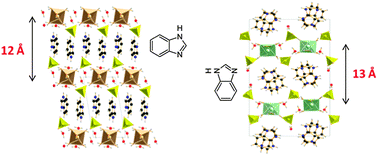
The use of benzimidazole as an aromatic diamine template in the synthesis of a series of hybrid double sulfate salts containing transition metals provided a new structure topology that gathers between the supramolecular aspect and the lamellar aspect, which is not very much encountered in the literature. The obtained materials with the general formula (C7H7N2)2[MII(H2O)6](SO4)2·4H2O (MII = Zn, Cu, Ni, Co) and (C7H7N2)2[Fe(H2O)6](SO4)2·3H2O crystallize according to the triclinic symmetry (S.G. P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) and to the monoclinic symmetry (S.G. P21/n), respectively. The mineral layer is built from sulfate (SO4), hexaaquametal-complex[MII(H2O)6] and free H2O molecules, linked together by hydrogen bonds only. The aromatic amines are intercalated between the inorganic layers and form chains through π⋯π interactions. The orientation of the amine molecule in two different directions can modify the interlayer space, which is able to vary from 12.3 to 13.2 Å depending upon on the nature of the metal. The thermal behaviour of the five metal compounds, studied by TDXD and TG, shows a good stability of precursors and indicates that the dehydration proceeds differently according to the metal incorporated into the structure leading to the formation of crystalline phases during heating. In particular, the Fe-related compound becomes amorphous when subjected to temperature increase.
) and to the monoclinic symmetry (S.G. P21/n), respectively. The mineral layer is built from sulfate (SO4), hexaaquametal-complex[MII(H2O)6] and free H2O molecules, linked together by hydrogen bonds only. The aromatic amines are intercalated between the inorganic layers and form chains through π⋯π interactions. The orientation of the amine molecule in two different directions can modify the interlayer space, which is able to vary from 12.3 to 13.2 Å depending upon on the nature of the metal. The thermal behaviour of the five metal compounds, studied by TDXD and TG, shows a good stability of precursors and indicates that the dehydration proceeds differently according to the metal incorporated into the structure leading to the formation of crystalline phases during heating. In particular, the Fe-related compound becomes amorphous when subjected to temperature increase.


 Please wait while we load your content...
Please wait while we load your content...