The synthesis of a manganese dioxide–iron oxide–graphene magnetic nanocomposite for enhanced uranium(vi) removal
Abstract
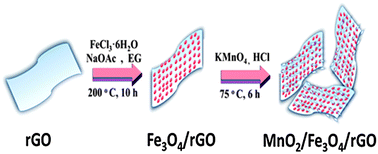
In this study, we have developed a facile route for the fabrication of a manganese dioxide–iron oxide–reduced graphite oxide magnetic nanocomposite (MnO2–Fe3O4–rGO). The as-obtained nanomaterial (MnO2–Fe3O4–rGO) was characterized using transmission electron microscopy, Fourier-transform infrared spectroscopy, X-ray diffraction, vibrating sample magnetometry, and Brunauer–Emmett–Teller surface area measurements. The MnO2–Fe3O4–rGO composite shows extraordinary adsorption capacity and fast adsorption rates for the removal of uranium(VI) in aqueous solution. The influence of factors including the dosage of the MnO2–Fe3O4–rGO composite used, pH of aqueous solution, and temperature were investigated. The thermodynamic parameters, including Gibbs free energy (ΔG°), standard enthalpy change (ΔH°) and standard entropy change (ΔS°) for the process, were calculated using the Langmuir constants. The results show that a pseudo-second-order kinetics model can be used to describe the uptake process using a kinetics test. Our present study suggests that the MnO2–Fe3O4–rGO composite can be used as a potential adsorbent for sorption of uranium(VI) as well as for providing a simple, fast separation method for the removal of uranium(VI) ions from aqueous solution.

 Please wait while we load your content...
Please wait while we load your content...