Microwave-assisted synthesis, molecular docking and antiproliferative activity of (3/5-aryl-1,2,4-oxadiazole-5/3-yl)(3,4,5-trimethoxyphenyl)methanone oxime derivatives†
Abstract
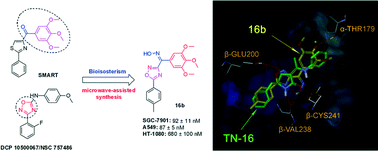
A series of (3/5-aryl-1,2,4-oxadiazole-5/3-yl)(3,4,5-trimethoxyphenyl)methanone oxime derivatives were synthesized via a rapid and facile microwave-assisted synthesis method of building a 1,2,4-oxadiazole skeleton using mandelic acid as the starting material. Twenty-four target compounds were evaluated for their in vitro antiproliferative activities against three human cancer cell lines (SGC-7901, A549 and HT-1080). Among them, 16b exhibited the highest potency against different tumour cell lines, especially the A549 cell line (IC50 = 87 nM). Structure–activity relationship (SAR) studies revealed that the aryl substituent at the C-5 position on the 1,2,4-oxadiazole ring is superior to that at the C-3 position. An oxime as a connector can obviously increase the potency, contrary to that in SMART derivatives. Moreover, 16b significantly induced a cell cycle arrest in the G2/M phase and caused microtubule destabilization. Molecular docking studies provided a theoretical binding mode of 16b at the colchicine site in the tubulin dimer. Our work laid the foundation for further structure-guided design of novel tubulin polymerization inhibitors.

 Please wait while we load your content...
Please wait while we load your content...