Determination of ultratraces of mercury species using separation with magnetic core-modified silver nanoparticles and electrothermal atomic absorption spectrometry
Abstract
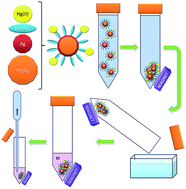
Magnetic particles covered with functionalized silver nanoparticles are used in a micro-solid phase extraction procedure for the separation and preconcentration of mercury compounds. Functionalization with the sodium salt of 2-mercaptoethane-sulphonate (MESNa) allows Hg(II) ions to be adsorbed. When MESNa is replaced by L-cysteine, monomethyl mercury, dimethyl mercury, ethyl mercury, phenyl mercury and diphenyl mercury are also retained. After separation by means of a magnetic field, the analytes are released from the nanocomposites by treating with a small volume of a potassium iodide solution. Mercury is then measured by electrothermal atomic absorption spectrometry using silver nitrate and potassium permanganate solutions for chemical modification. The preconcentration factor is close to 200, and a detection limit of 0.01 μg L−1 mercury is achieved. The results for total mercury are verified using certified reference materials. Water and edible fish oils are analyzed for total mercury and for two fractions or mercury organic compounds with the help of a solid phase extraction cartridge.

 Please wait while we load your content...
Please wait while we load your content...