Path to plastics composed of ligninsulphonates (lignosulfonates)
Abstract
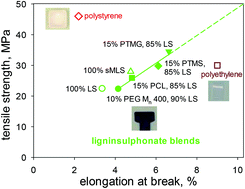
The properties of lignin-based polymeric materials depend on blend composition and casting conditions. When solution-cast at 150° and annealed at 180 °C, blends containing 85% w/w levels of methylated ball-milled softwood lignin can surpass polystyrene in tensile behaviour. It is more challenging to achieve similar results with comparable blends of ligninsulphonates, whether methylated or not. Nevertheless, 85% w/w ligninsulphonate-based materials containing miscible low-Tg polymers can match the tensile properties of polyethylene. The ligninsulphonate components themselves are assembled into 9–15 nm macromolecular entities, the integrities of which are maintained through strong noncovalent interactions between inner co-facially disposed aromatic rings. In contrast, the peripheral aromatic rings typically exhibit a greater incidence of less stable edge-on arrangements. An intriguing aspect of the materials that are composed solely of (unmethylated or methylated) ligninsulphonates is their insolubility in water. This observation is consistent with the absence of a discernible glass-transition temperature in the differential scanning calorimetric thermograms of ligninsulphonate-based materials when they prominently display domains maintained by strong interactions between co-facial aromatic rings. The demonstrated integrity of polymeric materials composed (almost) exclusively of simple lignin derivatives has far-reaching implications for the configurations of lignin macromolecules in general. Contrary to an enduring hypothesis proposed 55 years ago, polymer chains in lignin preparations are not covalently crosslinked, even though their conformations are clearly affected by powerful noncovalent forces between the aromatic substructures.
- This article is part of the themed collection: Lignin chemistry and valorisation

 Please wait while we load your content...
Please wait while we load your content...