CO2, COS and CS2 adducts of N-heterocyclic olefins and their application as organocatalysts for carbon dioxide fixation†
Abstract
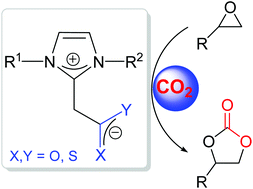
Various CO2, COS and CS2 adducts of N-heterocyclic olefins (NHOs) were synthesized and characterised by single crystal X-ray crystallography. The length of the C–O bond in NHO–COS adducts is slightly shorter than that in the corresponding NHO–CO2 adducts, while the C–S bond length in NHO–COS adducts is significantly longer than the corresponding C–S distance in the NHO–CS2 adducts, suggesting that the negative charge of the NHO–COS adducts is preferentially delocalized on the sulfur atom. The length of the CCS2–CNHO bond is significantly shorter than that of the CCOS–CNHO or CCO2–CNHO bond in the corresponding NHO–COS or NHO–CO2 adducts, implying the relatively high thermal stability of NHO–CS2 adducts. The NHO–CO2, NHO–COS and NHO–CS2 adducts were found to be efficient in catalyzing the cycloaddition reaction of CO2 and epoxides to selectively afford the corresponding cyclic carbonates. Among them, NHO–CO2 adducts were found to be more active, while NHO–CS2 adducts exhibited the lowest activity for this reaction, probably due to their high stability for difficult release of the highly active NHO.

 Please wait while we load your content...
Please wait while we load your content...