Anion exchange capacity of biochar†
Abstract
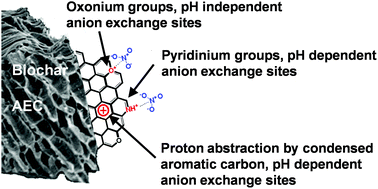
Biochar has gained recent interest as a soil amendment and agent for carbon sequestration. Some biochars have significant levels of anion exchange capacity (AEC), which may reduce leaching of anionic nutrients in soil. Little is known about the nature of anion exchange sites on biochar surfaces and what production conditions promote AEC in biochar. We report that the AEC of biochars produced from four feedstocks (maize stover, cellulose, alfalfa meal, and albumin) ranged from 0.602 to 27.76 cmol kg−1 and increased with decreasing pH (p < 0.0001) and peak pyrolysis temperature. A cellulose biochar, composed almost entirely of C, H, and O, exhibited significant AEC at pH 8 suggesting that pH independent O containing functional groups contribute AEC. Fourier transform infrared spectroscopy revealed a prominent 1590 cm−1 band, which we attribute in part to C–O+ stretching in oxonium heterocycles. Both the C1s and O1s X-ray photoelectron (XPS) spectra of the biochars provide additional evidence for oxonium heterocycles. The N1s XPS spectra of albumin biochars indicated the presence of pyridinic groups. We conclude that oxonium functional groups contribute pH independent AEC and that both pyridinic functional groups and non-specific proton adsorption by condensed aromatic rings contribute pH dependent AEC to biochars.

 Please wait while we load your content...
Please wait while we load your content...