Hydrodeoxygenation of lignin-derived phenols into alkanes over carbon nanotube supported Ru catalysts in biphasic systems†
Abstract
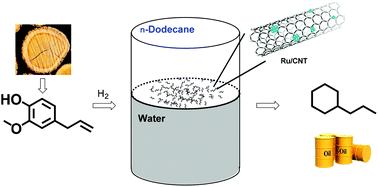
Phenolic compounds derived from lignin are important feedstocks for the sustainable production of alkane fuels with C6–C9 carbons. Hydrodeoxygenation (HDO) is the main chemical process to remove oxygen-containing functionalities. Here, we have reported the HDO of phenols in a biphasic H2O/n-dodecane system. A series of supported Ru catalysts were prepared, characterized and explored for the reaction among which Ru/CNT showed the highest catalytic activity towards the production of alkanes. The model reaction with eugenol achieved a high conversion (>99%) and a high alkane selectivity (98%), which was much higher than the results from the monophasic system (56.5% yield of alkanes in H2O). The reaction conditions including reaction temperature, hydrogen pressure and the ratio of H2O/n-C12H26 were optimized. The kinetic experiments revealed that eugenol was first hydrogenated to 4-propyl-guaiacol, and then deoxygenated into 4-propyl-cyclohexanol which was the main detected intermediate of the reaction. After that, 4-propyl-cyclohexanol was dehydrated and hydrogenated into propylcyclohexane. Moreover, various phenols and dimeric lignin model compounds were also successfully converted into alkanes in the biphasic systems. The construction of the biphasic solvent-Ru/CNT catalyst system highlights an efficient route for the conversion of lignin-derived phenolic compounds to biofuels.

 Please wait while we load your content...
Please wait while we load your content...