Dipyrrolylquinoxaline difluoroborates with intense red solid-state fluorescence†
Abstract
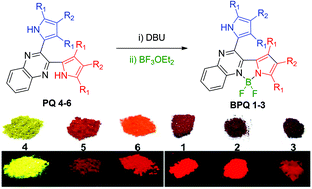
A set of organic fluorescent dyes of dipyrrolylquinoxalines (PQs 4–6) and their BF2 complexes (BPQs 1–3) were synthesized from commercial reagents, and were characterized by their X-ray structural analysis, and optical and electrochemical properties. BPQs 1–3 showed intense broad absorption in the visible region in the solution-state. In comparison with that of PQs 4–6, there is an over 110 nm red-shift of the absorption maximum in the BPQs 1–3 (up to 583 nm). Interestingly, dyes 1–6 all exhibit red solid-state fluorescence with moderate to high fluorescence quantum yields except for PQ 4 which showed bright yellow solid-state fluorescence. X-ray structures of BPQs 2–3 showed the planar structure of quinoxaline with one pyrrole unit via the BF2 chelation and the almost perpendicular orientation of the uncoordinated pyrrole to the NBN core plane (the dihedral angle of 70–73°). The extended π-conjugation was in good agreement with the observed red-shift of the spectra. These dyes formed well-ordered intermolecular packing structures via the intermolecular hydrogen bonding between the N atoms of quinoxaline moieties and the NH units of adjacent pyrroles. The lack of π–π stacking in their crystal packing structures may explain the interestingly intense solid-state fluorescence of these dyes.

 Please wait while we load your content...
Please wait while we load your content...