A helix–coil transition induced by the metal ion interaction with a grafted iron-binding site of the CyaY protein family†
Abstract
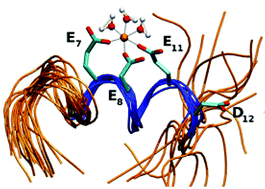
Iron–protein interactions are involved in electron transfer reactions. Alterations of these processes are present in a number of human pathologies; among them, in Friedreich's ataxia, in which a deficiency of functional frataxin, an iron-binding protein, leads to progressive neuromuscular degenerative disease. The putative iron-binding motif of acidic residues EExxED was selected from the first α-helical stretch of the frataxin protein family and grafted onto a foreign peptide scaffold corresponding to the C-terminal α-helix from E. coli thioredoxin. The resulting grafted peptide named GRAP was studied by applying experimental (circular dichroism, isothermal titration calorimetry, capillary zone electrophoresis, thermal denaturation, NMR) and computational approaches (docking, molecular dynamics simulations). Although isolated GRAP lacks a stable secondary structure in solution, when iron is added, the peptide acquires an α-helical structure. Here we have shown that the designed peptide is able to specifically bind Fe3+ with a moderate affinity (KD = 1.9 ± 0.2 μM) and a 1 : 1 stoichiometry. Remarkably, the GRAP/Fe3+ interaction is entropically driven (ΔH° = −1.53 ± 0.03 kcal mol−1 and TΔS° = 6.26 kcal mol−1). Experiments and simulations indicate that Fe3+ interacts with the peptide through three acidic side chains, inducing an α-helical conformation of the grafted motif. In addition, the acidic side chains involved undergo significant conformational rearrangements upon binding, as judged by the analysis of MDs. Altogether, these results contribute to an understanding of the iron-binding mechanisms in proteins and, in particular, in the case of human frataxin.

 Please wait while we load your content...
Please wait while we load your content...