On the effect of sodium salts on the coil-to-globule transition of poly(N-isopropylacrylamide)
Abstract
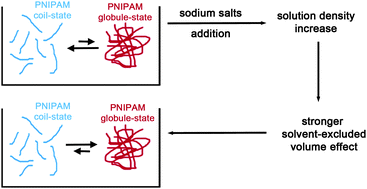
It has been shown that sodium salts significantly affect the temperature of the coil-to-globule collapse transition of poly(N-isopropylacrylamide) [J. Am. Chem. Soc., 2005, 127, 14505]. Since this phenomenon resembles the cold renaturation of globular proteins, it can be studied by means of the theoretical approach devised to rationalise the occurrence and the mechanism of cold denaturation [G. Graziano, Phys. Chem. Chem. Phys., 2010, 12, 14245; Phys. Chem. Chem. Phys., 2014, 16, 21755]. It emerges that the collapse transition is driven by the decrease in the solvent-excluded volume in order to maximise the translational entropy of water molecules and ions. At a given temperature, the aqueous solutions of sodium salts have densities higher than that of water. For this reason, the magnitude of the solvent-excluded volume effect proves to be larger, stabilizing the globular conformations of poly(N-isopropylacrylamide). On the other hand, two large ions, iodide and thiocyanate, are poorly hydrated and stabilise the coil conformations of the polymer by a preferential binding mechanism.

 Please wait while we load your content...
Please wait while we load your content...