Ternary B2X2H2 (X = O and S) rhombic clusters and their potential use as inorganic ligands in sandwich-type (B2X2H2)2Ni complexes
Abstract
Based upon global searches and electronic structure calculations at the B3LYP and CCSD(T) levels, we present the global-minimum structures of two ternary B–O–H and B–S–H rhombic clusters: D2h B2O2H2 (1, 1Ag) and C2v B2S2H2 (2, 1A1). Both species feature a B2X2 (X = O or S) four-membered ring as the core, with two H atoms attached terminally. The former cluster is perfectly planar, whereas the latter undergoes a slight butterfly distortion. Bonding analyses reveal a four-center four-electron (4c–4e) o-bond in these clusters, which are 4π systems in a nonbonding/bonding combination, in contrast to an antibonding/bonding combination in a classical 4π antiaromatic hydrocarbon such as cyclobutadiene (C4H4). Clusters 1 and 2 are considered to be aromatic. The present results also help elucidate the bonding nature in the relevant heteroatomic ring B2N2H4 system and suggest that it is not appropriate to consider B2N2H4 as an inorganic cyclobutadiene, a conception that has been in existence in the literature for over 40 years. The electronic properties of the global-minimum clusters 1 and 2 are predicted. It is shown that B2O2H2 (1) and B2S2H2 (2) may serve as effective inorganic ligands to form sandwich-type transition metal complexes, such as D2d [B2O2H2]2Ni (3) and D2d [B2S2H2]2Ni (4).
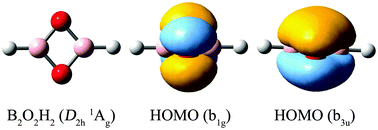

 Please wait while we load your content...
Please wait while we load your content...