Towards multielectron photocatalysis: a porphyrin array for lateral hole transfer and capture on a metal oxide surface†
Abstract
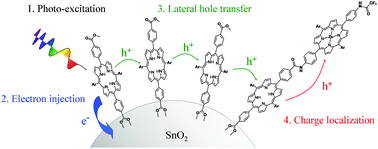
Current molecular water-oxidation photoelectrocatalytic cells have substantial kinetic limitations under normal solar photon flux where electron–hole recombination processes may outcompete charge buildup on the catalytic centers. One method of overcoming these limitations is to design a system where multiple light-harvesting dyes work cooperatively with a single catalyst. We report a porphyrin monomer/dyad array for analysis of lateral hole transfer on a SnO2 surface consisting of a free-base porphyrin that functions to absorb light and initiate charge injection into the conduction band of SnO2, which leaves a positive charge on the organic moiety, and a free-base porphyrin/Zn-porphyrin dyad molecule that functions as a thermodynamic trap for the photoinduced holes. By using transient absorption spectroscopy, we have determined that the holes on the surface-bound free-base porphyrins are highly mobile via electron self-exchange between close-packed neighbors. The lateral charge-transfer processes were modelled by treating the system statistically with a random-walk method that utilizes experimentally derived kinetic parameters. The results of the modelling indicate that each self-exchange (hop) occurs within 25 ns and that the holes are efficiently transferred to the Zn-porphyrin. This hole-harvesting scheme provides a framework for enhancing the efficiency of multielectron photoelectrocatalytic reactions such as the four-electron oxidation of water.

 Please wait while we load your content...
Please wait while we load your content...