Behaviour of NBD-head group labelled phosphatidylethanolamines in POPC bilayers: a molecular dynamics study†
Abstract
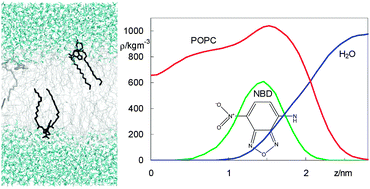
A complete homologous series of fluorescent phosphatidylethanolamines (diCnPE), labelled at the head group with a 7-nitrobenz-2-oxa-1,3-diazo-4-yl(NBD) fluorophore and inserted in 1-palmitoyl, 2-oleoyl-sn-glycero-3-phosphocholine (POPC) bilayers, was studied using atomistic molecular dynamics simulations. The longer-chained derivatives of NBD-diCnPE, with n = 14, 16, and 18, are commercially available, and widely used as fluorescent membrane probes. Properties such as location of atomic groups and acyl chain order parameters of both POPC and NBD-diCnPE, fluorophore orientation and hydrogen bonding, membrane electrostatic potential and lateral diffusion were calculated for all derivatives in the series. Most of these probes induce local disordering of POPC acyl chains, which is on the whole counterbalanced by ordering resulting from binding of sodium ions to lipid carbonyl/glycerol oxygen atoms. An exception is found for NBD-diC16PE, which displays optimal matching with POPC acyl chain length and induces a slight local ordering of phospholipid acyl chains. Compared to previously studied fatty amines, acyl chain-labelled phosphatidylcholines, and sterols bearing the same fluorescent tag, the chromophore in NBD-diCnPE locates in a similar region of the membrane (near the glycerol backbone/carbonyl region) but adopts a different orientation (with the NO2 group facing the interior of the bilayer). This modification leads to an inverted orientation of the P–N axis in the labelled lipid, which affects the interface properties, such as the membrane electrostatic potential and hydrogen bonding to lipid head group atoms. The implications of this study for the interpretation of the photophysical properties of NBD-diCnPE (complex fluorescence emission kinetics, differences with other NBD lipid probes) are discussed.

 Please wait while we load your content...
Please wait while we load your content...